BORON (B)
Boron is undoubtedly one of the most well-known and widely used trace elements in the world, as its deficiency can negatively affect plant growth, yield, and resistance to diseases. After years of continuous agricultural production, soils become depleted, and boron supplementation becomes necessary, especially for sensitive crops. Boron must be applied carefully and in controlled doses, as excess boron can be just as harmful as deficiency.
B
IMPORTANCE FOR PLANT LIFE
METABOLISM
Boron plays a role in the conversion of nitrate into amino acids, thereby contributing to protein synthesis. It also increases the thickness and strength of cell membranes, enhancing the mechanical resistance of plant tissues.
GROWTH
Boron plays a key role in carbohydrate and protein synthesis and is essential for the growth of plant cells and tissues. It is required for cell division, new tissue formation, and the healthy development of meristematic regions.
YIELD
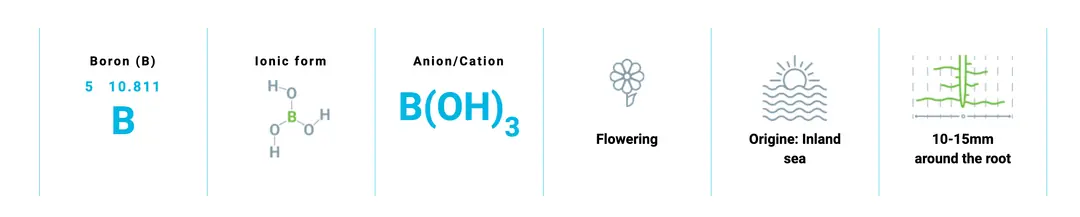
Boron is involved in the formation of reproductive cells (especially pollen) and therefore directly affects flowering, fertilization, and grain or fruit set. Adequate boron improves flower and fruit retention, contributing to higher yields.
ABSORPTION MECHANISMS
Plants absorb boron from the soil solution in the form of soluble boric acid. Uptake is proportional to water absorption and is therefore considered passive. Since boron mobility within the plant is limited, higher concentrations often accumulate in older leaves, while newly formed young leaves may contain lower levels of boron.
INTERACTIONS AND SPECIFIC CHARACTERISTICS
Boron availability to plants depends on the exchangeable boron reserve in the field, seasonal and climatic conditions (especially high rainfall causing leaching), soil biological activity, and the physiological demand of the crop.
Crop species and varieties also influence boron uptake and demand; some species and cultivars are more sensitive to boron and require higher boron levels.
BORON IN SOIL
The natural boron supply in soils largely depends on soil type and parent material. If the parent rock is of magmatic origin, boron content is generally very low. Sedimentary rocks and marine environments where these rocks formed are richer in boron.
Similar to potassium fertilizers, boron can be retained by clay layers and may re-enter the soil solution through periodic changes in soil moisture (wet–dry cycles).
In acidic soils, iron and aluminum ions, and in alkaline soils calcium, can bind boron and reduce its plant-available form. In addition to these physicochemical mechanisms, the incorporation of organic materials naturally replenishes soil boron levels.
In farming systems without organic fertilizer use, mineral boron fertilizers are required to compensate for boron removed by harvest; otherwise, soil boron reserves gradually decline.
SENSITIVITY & SYMPTOMS
Boron deficiency in plants manifests as chlorosis (yellowing), deformities, and necrosis (tissue death) in certain plant parts. Deformations at growing points and young tissues, as well as problems in flower and fruit development, are commonly observed.
EXCESS & REQUIREMENT
Boric acid is a strong bactericide. Even in crops with high boron demand, such as oilseed rape (canola), excessive boron application should be avoided, as boron toxicity can negatively affect yield and cause toxic symptoms.
ORIGIN & PRODUCTION
NATURAL ORIGINS
Boron accumulates naturally in specific regions through a complex geological cycle, which is why boron deposits are limited worldwide. This process occurs in two main stages:
1) Long-term deposition in inland areas, during which boron becomes concentrated through gases escaping from fumaroles.
2) Redissolution and concentration in a warm, enclosed inland sea exposed to evaporation, leading to the precipitation of boron as calcium or sodium borates on the seabed.
PRODUCTION PROCESS
From an industrial perspective, the main objective is to formulate ready-to-use boron products with the appropriate solubility level to ensure high nutrient efficiency for plant nutrition.
LAT Nitrogen uses two production methods for this purpose:
• The first method dissolves boron using acid and then complexes it
within an organic molecule to prevent rapid degradation.
• The second method micronizes boron to a sufficiently fine particle
size, enabling boron particles to penetrate the leaf surface during
foliar application.
KEY FACTORS
SOIL AND ORGANIC MATTER CONTENT
Hot-water extraction is a widely accepted method for measuring boron in soils. The following minimum reference values can be used:
- At least 0.8 ppm boron in calcareous soils
- At least 0.6 ppm boron in neutral soils
- At least 0.4 ppm boron in acidic soils
A significant portion of soluble boron originates from organic matter. Low organic matter content limits boron availability in the soil solution. An organic matter level below 1.8% is considered critical in terms of boron deficiency risk.
TEXTURE
In clay soils, boron tends to be complexed and retained within clay layers; however, under suitable conditions, it can relatively easily return to the soil solution. In contrast, sandy soils retain little boron and are therefore much more prone to leaching.
CLIMATE
Rainy periods increase boron leaching, while dry periods hinder boron dissolution. As a result, boron availability in the soil solution is generally lower in arid regions, whereas leaching risk is higher in humid regions.
pH
pH is one of the most important factors determining boron availability to plants. As soil pH increases, boron availability decreases and deficiencies become more frequent. When pH exceeds 6.5, boron uptake declines significantly. Therefore, liming practices can negatively affect boron availability and must be carefully planned.
